Cancer patients expressed their relief at being able to continue taking life-saving medications as a result of a policy change.
In England, doctors were not permitted to automatically re-prescribe two medications for bowel cancer if a patient had stopped taking them.
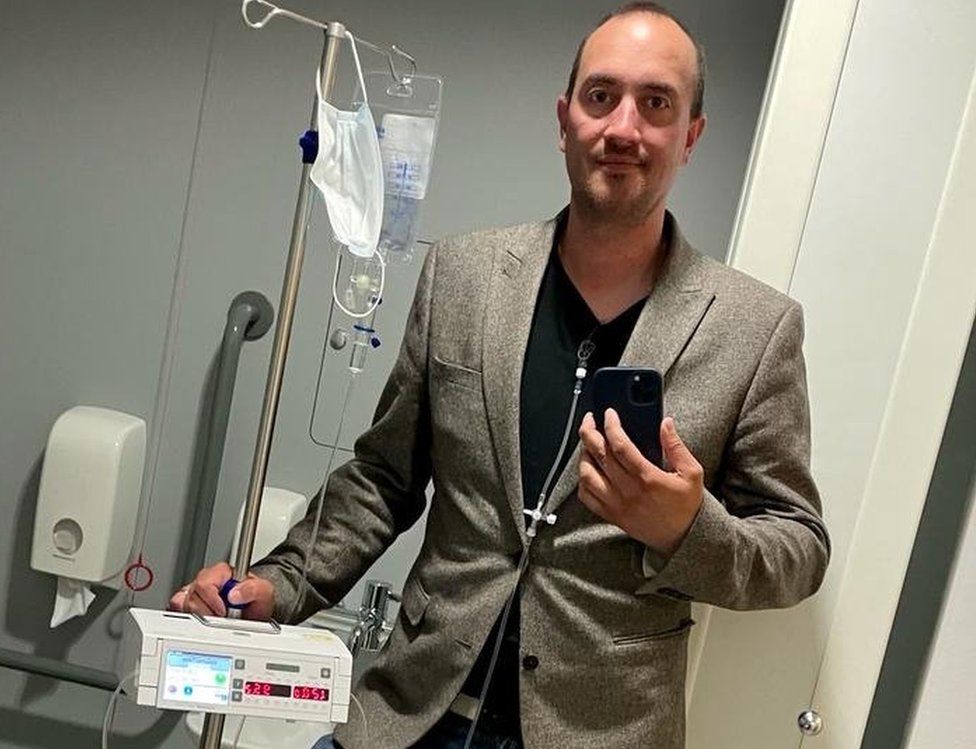
The decision, according to Brantham, Suffolk resident Steven Marsland, 38, meant he now had "one less thing to worry about.".
Cetuximab and panitumumab were impacted by the policy.
Prior to the rule change, patients who took a treatment break of more than six weeks had to reapply for NHS funding, with no assurance that their application would be accepted.
Mr. Marsland, who has an incurable cancer, had to put his treatment on hold last year so he could have surgery for a hernia. Because of the policy, he was forced to take a shorter break and worried that in the future he might have to pay for cetuximab himself.
Since his diagnosis in 2018, the father-of-two, a principal technologist for BT, has undergone 93 rounds of chemotherapy and 28 lots of radiotherapy.

I no longer have to worry about losing a line of treatment, he said, thanks to this choice.
"It will give people more options for skin recovery breaks, vacations, and mini-surgeries. I won't have to conceal illnesses out of concern for being exposed. ".

Jane Ashford, a resident of Bristol, called the development "amazing" and said she had endured "constant fear" of having her treatment stopped.
The 50-year-old woman, who has bowel cancer that is currently in remission, claimed that her oncologist wanted her to take a break because the drug toxicity was causing serious health problems, but that she was forced to continue with no breaks because of the arbitrary rule.
She claimed that the adverse physical, emotional, and psychological effects forced her to resign from her position as a lead nurse specialist for the NHS.
For some patients, the rule modification came too late. After taking a break for liver surgery, Roy Davison, a father-of-three from Preston, was informed that he could not resume taking panitumumab on the NHS.

According to his wife Carolyn, the engineer, who was diagnosed with bowel cancer in 2014, was making good progress and that the drug had caused his tumors to become operable.
"Then the doctors informed us that the guidelines had changed, and the medication was abruptly taken away. It appeared to be both cruel and wrong.
It was as though he was only permitted to take half of a prescription that had been written. Oncologists who provided second opinions said they would all like to restart him on panitumumab if they could, she said.
They wrote to the drug maker and their MP to explain the situation, but Mr. Davison was compelled to take other medications, which caused his cancer to worsen. In 2017, he passed away at the age of 59.
Bowel Cancer UK, which has been fighting the disease for five years, has a chief executive named Genevieve Edwards. She said: "Patients with advanced bowel cancer have very few treatment options, and these medications are frequently their only lifeline.
"This decision by NHS England will give patients with advanced bowel cancer new hope for a better quality of life, more time with loved ones, and, for some, the possibility of a full remission. ".
For a response, NHS England has been contacted.